Antisense RNA regulation in Helicobacter pylori
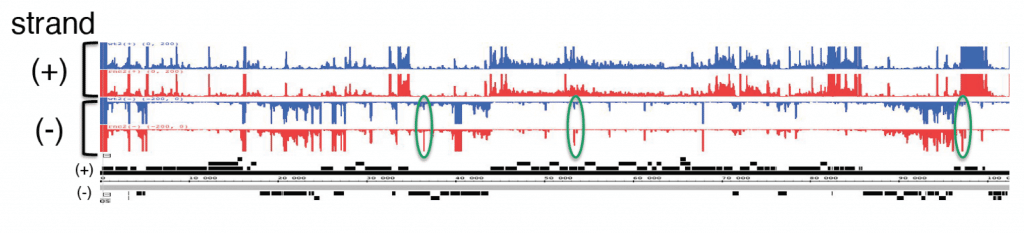
The human bacterial pathogen Helicobacter pylori (H. pylori) is the major cause of chronic gastritis and peptic ulcers, and is associated with malignant stomach diseases. About half of the world’s population is infected with this Gram-negative spiral shaped bacterium from the epsilon-proteobacteria group. H. pylori colonizes the stomach and copes with many stresses encountered during infection, e.g., pH and nutrient fluctuations. Differential RNA-seq analysis of the primary transcriptome of the human pathogen H. pylori revealed hundreds of cis-encoded sRNAs (Sharma et al., 2010). Whereas most of these newly identified “antisense” transcripts overlap mRNAs, some of them overlap stable RNAs such as ribosomal RNA or tRNA. Our objective is to determine whether these antisense RNAs influence the expression of their sense-strand targets and to study the underlying mechanism. We also study the regulation of these sRNAs by ribonucleases and analyse their biological functions.
Exploring Toxin Antitoxin systems in epsilon proteobacteria
Toxin-antitoxin (TA) systems are small genetic modules composed of a toxin and an antitoxin gene, present in almost all bacterial genomes including those of bacterial pathogens. We recently reported that the genome of H. pylori is hosting several copies of a new family of TA system. Similarly to other type I TA systems, the toxin is a small membrane protein whose production is inhibited by a small antisense RNA (antitoxin) that binds to the 5’ untranslated region of the toxin mRNA. A search based on structural homology identified hundreds of homologs of this new TA system on the chromosome of other Helicobacter (see figure bellow) and Campylobacter species. This TA system named AapA/IsoA constitutes a new family of type I TA system outside enterobacteria and firmicutes.
Currently our research project aims at understanding how type I TA systems are regulated in bacterial cells and how H. pylori copes with their presence in its chromosome. In collaboration with chemists (Dr. M. Duca, Institute of Chemistry, Nice, France), we are also exploring how these systems could be used to develop new antibiotic strategies.
MiRNAs, H. pylori and Gastric Cancer
MicroRNA (miRNA) are small eukaryotic noncoding RNAs playing prominent roles in the control of cell proliferation and differentiation. Deregulation of their expression is associated with pathological states, including cancer, immune disorders and infection. Acting at the post-transcriptional level, miRNAs have expanded our understanding of the control of gene expression in regulatory networks involved in the adaptation to environmental situations such as biotic stress and host response to microbes (see our review, Staedel & Darfeuille 2013).
Kinetic of infection of epithelial gastric cells (AGS cells) by H. pylori. Four hours after the inoculation of the bacterium, we observe the formation of the « humming bird » phenotype which corresponds to an epithelial to mesenchymal transition (credit: C. Staedel, INSERM).
For instance, some microRNAs like miR-146a and -155 are commonly affected during bacterial infection and contribute to immune responses protecting the organism against overwhelmed inflammation (Hoches de la Guardia et al, 2013). Besides, cell-specific relationships between miRNAs and their targets are also engaged in the alterations of proliferation/differentiation/apoptosis pathways. Having characterized the miRNome of a human gastric epithelial cell model in response to a virulent H. pylori strain (see miRNA expression pattern above), we found that the abundant miR-372 and -373 are negatively regulated during infection, subsequently de-repressing their common target, the cell cycle regulator LATS2 and blocking cell cycle progression (Belair et al. 2011). In parallel, the infected cells undergo an epithelial-to-mesenchymal transition (EMT): the EMT inducer ZEB1 and the miR-200b&c, engaged in a reciprocal negative feedback loop, are both up-regulated by the infection-activated transcription factor NFkB. The enhanced miR-200 levels thwart the irreversible loss of epithelial identity in that critical situation (Baud et al. 2013). The H. pylori-induced mesenchymal phenotype of gastric epithelial cells confers invasive, tumorigenic, and cancer-stem cell properties (Bessède et al. 2013). Our results highlight several markers of the H. pylori-infected gastric mucosa revealing pre-neoplasic lesions at atrophic gastritis and intestinal metaplasia stages. Now our projects aim at developing miRNA inhibitors using two strategies: a) by blocking its biosynthesis at the pre-miRNA level using small RNA ligand (Vo et al. 2014), b) by sequestering its mature form with a 8-mer self-penetrating antisense locked-nucleic acid (Staedel et al., 2015).


